By Joseph Staples // SWNS
NEWS COPY W/ VIDEO + INFOGRAPHIC
Is what’s on your wrist more valuable to your health than what’s in your medicine cabinet?
A new survey of 2,000 Americans revealed consumer sentiments about purchasing, using, and relying on medical devices for vital health information sharing.
More than one in four consumers (28%) reported they have had a personal medical device alert them to a pending health issue.
When alerted, 84% found the data “extremely” or “very” valuable, and 76% said they’ve had their health issue successfully diagnosed after consulting with a doctor.
Commissioned by Propel Software and conducted by Talker Research, the study found 80% of consumers own at least one medical device, including: blood pressure monitors (45%), electric toothbrushes (39%), fitness trackers and pedometers (24%), smartwatches or smart rings (23%) and blood-glucose monitors (18%).
However, many were unaware of the scope of medical devices available today and their benefits.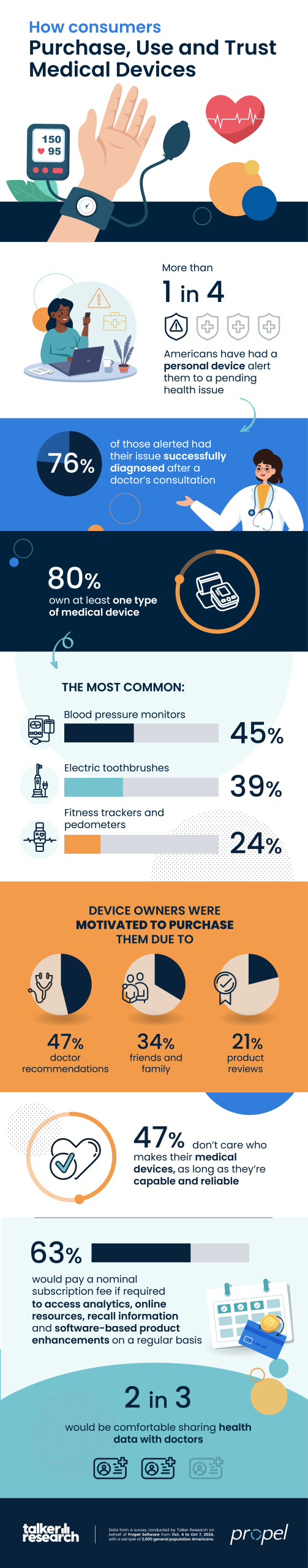
While 40% correctly identified medical devices as “any device that can track health information,” 27% believe medical devices are “any product that interacts with their body,” and another 20% believe they’re “devices that are specifically designed and used in hospital settings.”
The majority of respondents were aware of certain medical devices — blood pressure monitors (88%), heart rate monitors (86%) and blood-glucose monitors (84%).
But they were unaware that some everyday devices like electric toothbrushes (33%), baby monitors (20%) and smartwatches/smart rings (18%) also fall under the definition of a medical device.
Those who own medical devices said they were motivated to purchase their devices due to recommendations from their doctor (47%), friends and family (34%) and product reviews (21%).
“The knowledge and speed at which these devices – from blood pressure and glucose monitors to baby monitors – provide consumer feedback continues to accelerate, and it’s fascinating to witness how medical device manufacturers are quickly adapting to market and consumer feedback and preferences. At the same time, consumer product companies are increasingly entering the medical device market,” said Chuck Serrin, Vice President Industry Marketing, Medical Device & Life Sciences at Propel Software. “Tech-savvy consumers embracing the convenience and real-time health alerts of these devices will forever change how we all think about our personal health care.”
The study also found many purchase decisions were influenced by insurance recommendations and coverage (39%), doctor testimonials (38%) and FDA approval (36%).
In addition, 63% of consumers reported they would likely pay a nominal subscription fee if required to access analytics, online resources, recall information, and software-based product enhancements on a regular basis.
Two in three (68%) also said it was key that their medical devices have a consumer-friendly appearance — looking inconspicuous and designed for everyday wear and use.
When it comes to data information sharing, two in three respondents stated they’d be comfortable with their devices either sharing information over the internet directly with their general practitioner (62%) or with a specialist they’ve met before (66%).
Both women (61%) and men (63%) reported feeling comfortable sharing health feedback from their device online with medical practitioners.
“Surprisingly, brand loyalty was not top of mind with medical device consumers — 47% claim they don’t have a ‘brand’ preference as long as the device is capable and reliable,” Serrin added. “However, consumers reported that product reviews and recommendations greatly influence purchasing decisions.
“With 71% reporting devices were more trustworthy when accompanied directly by doctors’ office reviews, it’s essential that device manufacturers get products right so favorable reviews are posted quickly to influence sales.”
WHAT DEVICES DO AMERICANS OWN?
- A blood pressure monitor - 45%
- An electric toothbrush - 39%
- Fitness tracker/pedometer - 24%
- A smartwatch/ring - 23%
- A blood-glucose monitoring device - 18%
- A heart rate monitor - 14%
- A blood-oxygen monitoring device - 13%
- A sleep tracking device - 11%
- A CPAP machine - 11%
- Hearing aids - 8%
- Baby monitoring smart devices - 5%
Survey methodology:
Talker Research surveyed 2,000 general population Americans; the survey was commissioned by Propel Software and administered and conducted online by Talker Research between Oct. 4 and Oct. 7, 2024.
We are sourcing from a non-probability frame and the two main sources we use are:
- Traditional online access panels — where respondents opt-in to take part in online market research for an incentive
- Programmatic — where respondents are online and are given the option to take part in a survey to receive a virtual incentive usually related to the online activity they are engaging in
Those who did not fit the specified sample were terminated from the survey. As the survey is fielded, dynamic online sampling is used, adjusting targeting to achieve the quotas specified as part of the sampling plan.
Regardless of which sources a respondent came from, they were directed to an Online Survey, where the survey was conducted in English; a link to the questionnaire can be shared upon request. Respondents were awarded points for completing the survey. These points have a small cash-equivalent monetary value.
Cells are only reported on for analysis if they have a minimum of 80 respondents, and statistical significance is calculated at the 95% level. Data is not weighted, but quotas and other parameters are put in place to reach the desired sample.
Interviews are excluded from the final analysis if they failed quality-checking measures. This includes:
- Speeders: Respondents who complete the survey in a time that is quicker than one-third of the median length of interview are disqualified as speeders
- Open ends: All verbatim responses (full open-ended questions as well as other please specify options) are checked for inappropriate or irrelevant text
- Bots: Captcha is enabled on surveys, which allows the research team to identify and disqualify bots
- Duplicates: Survey software has “deduping” based on digital fingerprinting, which ensures nobody is allowed to take the survey more than once
It is worth noting that this survey was only available to individuals with internet access, and the results may not be generalizable to those without internet access.




